Why is too much sugar bad for us?
Anoushka Sharp
Before we start, there’s two things to say.
1) ‘Too much’ of any type of food – not just sugar – is bad for you. This article is about eating too much sugar because it’s a far commoner problem than eating too much cucumber and spinach.
2) ‘Too much’ will be a different amount for different people – as determined by their genetics, environment and lifestyle.

Now, we’re ready to start – why is too much sugar bad for you? Let’s break down the question into two simpler parts: ‘where in the body does the excess sugar go?’ and ‘what bad stuff does this sugar do when it gets there?’
Part one: where does the excess sugar go?
Normally, sugar that you eat is broken down into glucose, which travels from your small intestine into your bloodstream and then into your cells.
Eating too much sugary food means that some excess glucose stays in the blood for longer than normal.
Most excess glucose taken up by body cells. There, glucose is broken down to create ATP (Adenosine TriPhosphate, immediate energy for cells to perform chemical reactions) or converted into long-term energy stores. Glucose can be stored as Glycogen – a chemical made of lots of glucose molecules stuck together. Alternatively, Glucose can be broken down to create excess ATP (energy), and this energy is used to build up fat stores from fatty acids. This is shown below.
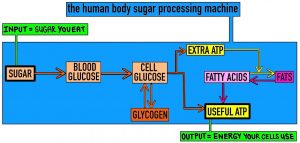
Just a sidenote… it’s a very strange machine! Usually, you input something into a machine and get an output that depends on the input. For example, if I put a bigger potato in the microwave as my input, I will eat a bigger potato as my output. . A similar machine structure is found in your mobile phone. You choose how much to charge the phone battery (energy input), and you choose how much to use the phone (energy output). The phone will respond to your choices by storing some energy in its battery and using the rest to send Whatsapp messages or watch Youtube.
Part two: what bad stuff does sugar do in the body?
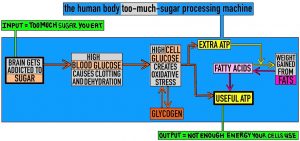
In the image above, grey boxes represent extra sugar wreaking havoc in the body. Below is a short explanation of each box.
The brain box:
Addiction to sugar can happen if you eat too much because there are some neurons in your brain that make you feel happy when you taste sugary stuff or when your blood glucose gets high after having been low. If you eat a continuously high level of sugar, your neurons stop noticing the sugar so much (this is called receptor desensitisation) so you have to eat sweeter foods in larger amounts for your brain to notice them. Your brain is unhappy about receptor desensitisation because the sensation of eating sugar is nice. You crave the sweeter foods in larger amounts that will stimulate your desensitised neurons.
The blood box:
Glucose is very sticky (which causes blood clotting) and attracts water (so body cells become dehydrated as water is sucked out of cells towards glucose in the blood). These properties of glucose – stickiness and water-attractiveness – are due to chemical structure: each glucose molecule forms lots of hydrogen bonds.
What is a hydrogen bond?
It’s a weak chemical bond between two molecules, making them stick together without combining to make a larger molecule. Hydrogen bonds are made if a hydrogen atom in one molecule attracts an oxygen or nitrogen atom in a neighbouring molecule. If you were a molecule making a hydrogen bond with your friend, another molecule, you would do it by holding hands: joining firmly together without becoming a single person! Glucose molecules can form hydrogen bonds with molecules of water (allowing glucose to dissolve in water), or molecules of solid surfaces that glucose sticks to. For example, a sticky cake containing glucose would stick to the cake tin because glucose molecules in the cake form hydrogen bonds with molecules of ‘cake-tin’. Glucose is stickier than water (which also makes hydrogen bonds) because a glucose molecule makes more hydrogen bonds than a water molecule.
The cell box
‘Oxidative stress’ means ‘free radicals harm the cell’. Free radicals are molecules that contain an odd number of electrons. Free radicals are unstable (i.e. they don’t last for long before performing chemical reactions) because electrons usually hang around in pairs. The ‘unpaired’ electron in a free radical can break important chemical bonds in a cell’s DNA, which increases the risk of cancer. Free radicals are created in small amounts every time a molecule of glucose is broken down. I imagine free radicals as desperately lonely people who disrupt the structure of existing friendship groups in order to make friends.
The fat box
As mentioned before, excess ATP gained from breaking down excess glucose is used to build up stores of fat. That’s because each cell can only store a limited amount of glycogen, but the body can store a practically limitless amount of fat by creating new cells, called adipocytes, which only have one function – to store fat! During weight gain (from fat), new adipocytes are created and existing adipocytes get larger. During weight loss (of fat), existing adipocytes release their stores of fat into the bloodstream. If an adipocyte has released all of its fat, it commits apoptosis (i.e. dies quickly and quietly without causing any local damage).
Conclusion: are you terrified of chocolate now?
I hope not! As shown in the first image, many people – especially if they are very active – can eat sugary food in moderation without any detriment to their health. The world’s best chocaholic (i.e. someone who loves dessert) knows how to enjoy eating sugar without getting addicted to it or eating too much. The world’s best chocaholic might eat a biscuit after breakfast, two gummy bears after lunch, and three Maltesers before dinner – but the world’s worst too-much-chocaholic would try to eat three Maltesers before dinner, realise that Maltesers are actually quite yummy, finish three packets of Maltesers, and then not eat dinner.
 Good luck in your quest to find enjoyment without addiction, and bonne appetite for your dessert!
Good luck in your quest to find enjoyment without addiction, and bonne appetite for your dessert!
